- 유관기관
- 전북대 산학협력단
- 서울대 반도체공동연구소
- 경북대 반도체공정교육센터
- 연구기관
- ETRI(한국전자동신연구원)
- 한국전기연구원
- 한국광기술원
- 나노종합기술원
- 한국나노기술원
- 관련정부부처 및 재단
- 교육부
- 과학기술정보통신부
- 정보통신산업진흥원
- 산업통상자원부
- 한국연구재단
- 한국산업기술평가관리원
Research Highlight
- Home
- TECHNOLOGY
- Research Highlight
2008~2012
-
2008
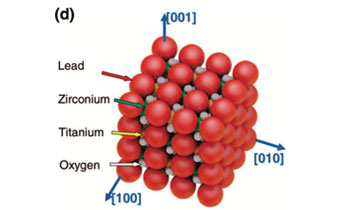
Ferroelectricity in highly ordered arrays of ultra-thin-walled Pb (Zr, Ti) O3 nanotubes composed of nanometer-sized perovskite crystallitesKim, J., Yang, S. A., Choi, Y. C., Han, J. K., Jeong, K. O., Yun, Y. J., ... & Bu, S. D. (2008), Nano Letters, 8(7), 1813-1818.
We report the first unambiguous ferroelectric properties of ultra-thin-walled Pb(Zr,Ti)O3 (PZT) nanotube arrays, each with 5 nm thick walls and outer diameters of 50 nm. Ferroelectric switching behavior with well-saturated hysteresis loops is observed in these ferroelectric PZT nanotubes with Pr and Ec values of about 1.5 μC cm-2 and 86 kV cm-1, respectively, for a maximum applied electric field of 400 kV cm-1. These PZT nanotube arrays might provide a competitive approach toward the development of three-dimensional capacitors for the terabyte ferroelectric random access memory.
-
2010
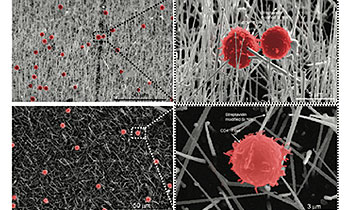
Novel streptavidin-functionalized silicon nanowire arrays for CD4+ T lymphocyte separationKim, S. T., Kim, D. J., Kim, T. J., Seo, D. W., Kim, T. H., Lee, S. Y., ... & Lee, S. K. (2010), Nano Letters, 10(8), 2877-2883.
Silicon nanowires (SiNWs) offer promising inorganic nanostructures for biomedical application. Here, we report the development of a novel SiNW array designed for isolating primary CD4+ T lymphocytes from the heterogeneous mixture of cell populations. Our system employed the specific high-affinity binding features of streptavidin (STR)-functionalized SiNW with biotinlabeled CD4+ T lymphocytes. Fabricated SiNW arrays easily separated the CD4 T lymphocytes from the mouse whole splenocytes with over ∼88% purity and demonstrated tight attachment to CD4+ T lymphocytes by scanning electron microscopy. Thus, our STR-SiNW arrays provide a potential tool for specific cell separation and further present a possibility to be applied to the other area of biomedical applications.
-
2012
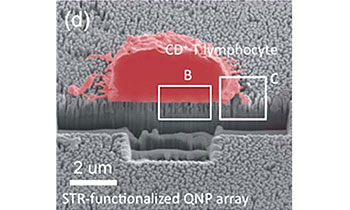
A quartz nanopillar hemocytometer for high-yield separation and counting of CD4+ T lymphocytesKim, D. J., Seol, J. K., Wu, Y., Ji, S., Kim, G. S., Hyung, J. H., ... & Lee, S. K. (2012), Nanoscale, 4(7), 2500-2507.
We report the development of a novel quartz nanopillar (QNP) array cell separation system capable of selectively capturing and isolating a single cell population including primary CD4+ T lymphocytes from the whole pool of splenocytes. Integrated with a photolithographically patterned hemocytometer structure, the streptavidin (STR)-functionalized-QNP (STR-QNP) arrays allow for direct quantitation of captured cells using high content imaging. This technology exhibits an excellent separation yield (efficiency) of 95.3 1.1% for the CD4+ T lymphocytes from the mouse splenocyte suspensions and good linear response for quantitating captured CD4+ T-lymphoblasts, which is comparable to flow cytometry and outperforms any non-nanostructured surface capture techniques, i.e. cell panning. This nanopillar hemocytometer represents a simple, yet efficient cell capture and counting technology and may find immediate applications for diagnosis and immune monitoring in the point-of-care setting.
-
2012
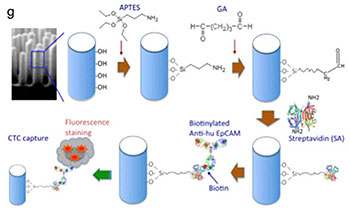
Nanowire substrate-based laser scanning cytometry for quantitation of circulating tumor cellsLee, S. K., Kim, G. S., Wu, Y., Kim, D. J., Lu, Y., Kwak, M., ... & Fan, R. (2012), Nano Letters, 12(6), 2697-2704.
We report on the development of a nanowire substrate-enabled laser scanning imaging cytometry for rare cell analysis in order to achieve quantitative, automated, and functional evaluation of circulating tumor cells. Immuno-functionalized nanowire arrays have been demonstrated as a superior material to capture rare cells from heterogeneous cell populations. The laser scanning cytometry method enables large-area, automated quantitation of captured cells and rapid evaluation of functional cellular parameters (e.g. size, shape and signaling protein) at the singlecell level. This integrated platform was first tested for capture and quantitation of human lung carcinoma cells from a mixture of tumor cells and leukocytes. We further applied it to the analysis of rare tumor cells spiked in fresh human whole blood (several cells per mL) that emulate metastatic cancer patient blood and demonstrated the potential of this technology for analyzing circulating tumor cells in the clinical settings. Using a high-content image analysis algorithm, cellular morphometric parameters and fluorescence intensities can be rapidly quantitated in an automated, unbiased, and standardized manner. Together, this approach enables informative characterization of captured cells in situ and potentially allows for sub-classification of circulating tumor cells, a key step towards the identification of true metastasis-initiating cells. Thus, this nano-enabled platform holds great potential for studying the biology of rare tumor cells and for differential diagnosis of cancer progression and metastasis.